Evaluating Microbial Urease Activity
In this laboratory we will be able to assemble a simple enzymatic assay to detect the activity of the enzyme urease, which you will be able to keep and test by yourself with samples from your school, home, and neighborhood. Many microbes synthesize urease to help them obtain nitrogen from the ammonia produced in the hydrolysis of urea. Urease is the protein that accelerates that reaction according to the following equation:
(NH2)2CO(aq)   +  H2O(l)      ->        CO2(g)      +    2NH3(g)
Urea                       Water           Carbon dioxide       Ammonia
Ammonia is an alkaline gas that can easily be detected by color change of a pH indicator, so we will assemble a colorimetric test to detect ammonia, which indicates the presence of urease should urea is used as a reaction substrate.
Materials
- Paper with urea (white paper)
- Paper with pH indicator (yellow paper)
- Plastic tubes with caps
- Hole puncher
- Tweezers
- Standard solutions of urease and droppers to test the assembled assays
Test assemblage and evaluation
-
You will be handed two different papers that contain either urea (white paper) or a pH indicator (yellow paper). Using the hole puncher, pierce several holes in both papers and collect the small paper circles.
- Place a yellow paper circle in the interior of a tube’s cap, so that the paper remains stuck and is visible from the external side of the cap.
- Place a white paper circle inside a tube containing a yellow paper circle in its cap. Close the tube. The figure below shows where the paper should be located.
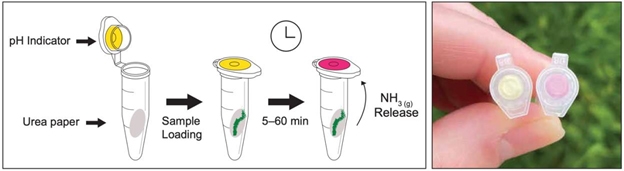
- Repeat with other tubes and prepare as many as you want. Remember that you get to keep these, so you can test it at home!
- Test your assay by introducing a small drop of urease standard solution inside the tube, making sure the sample is in contact with the white paper, but not the yellow paper. Close the tube and wait for a few minutes to see a color change.
- Record the time from the introduction of your sample until you start to see a change in color in the indicator from yellow to red. Different solutions will change the color at different times, so you have to find out which one is faster!
Evaluation of environmental samples
The tests you just assembled could be used to detect the presence of urease from microbes in diverse environments. Simply put a small, wet sample in contact with the white paper, close the tube, and record the time if you see any changes in color compared to an unused tube.
Remember that microbes are everywhere! Examples of samples that could be evaluated are:
- Slime from rocks that are wet, usually underwater in pounds and streams
- Green filaments from pounds and streams
- Wet soil and water
In case that you find samples that are positive, meaning that the assay turns red in less than
~1h, take note of the site and you could let us know by contacting us at (or even if you don’t find positive samples, we want to know what did you find!). Maybe you just found an important site that could result in a relevant scientific discovery!
References
The enzymatic assay used for this activity was based on:
Medina Ferrer F, Hobart K, Bailey JV. (2020) Field detection of urease and carbonic anhydrase activity using rapid and economical tests to assess microbially induced carbonate precipitation. Microbial Biotechnology 13(6):1877‚Äď1888.
Learn how microbial urease could be used to produce ‚Äėbioconcrete‚Äô and how this assay helped determine the way microbes build certain rocks:
Castro-Alonso MJ, Monta√Īez-Hernandez LE, Sanchez-Mu√Īoz MA, Macias Franco MR, Narayanasamy R, Balagurusamy N. (2019) Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Frontiers in Materials 6:126.
Medina Ferrer F, Rosen MR, Feyhl-Buska J, Russell VV, S√łnderholm F, Loyd S, Shapiro R, Stamps B, Petryshyn V, Demirel-Floyd C, Bailey JV, Johnson H, Spear J, Corsetti F. (2022) Potential role for microbial ureolysis in the rapid formation of carbonate tufa mounds. Geobiology 20(1):79‚Äď97.